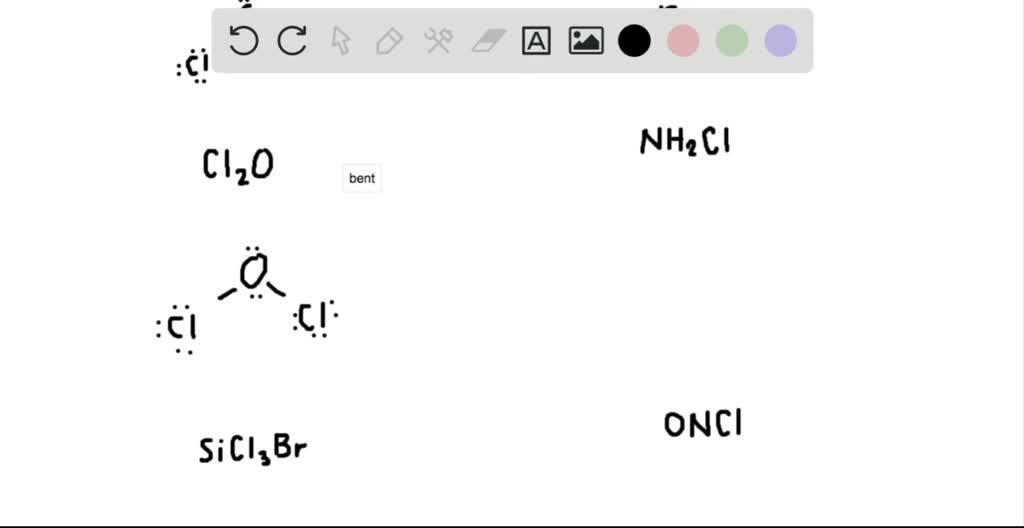
1. [PDF] KEY
193 #2: (6) Draw the Lewis structure, and use the VSEPR theory to name the geometry of the following molecules: a) SCI2 b) NH₂Cl c)SiCl3Br. CI: : CI: :C1: H.
2. What is the Lewis structure for SiCl2Br2? - Answers
Apr 15, 2009 · The Lewis structure for SiCl2Br2 is where silicon is in the center, surrounded by two chlorine atoms and two bromine atoms. Each halogen atom ...
LOLOLOLOLOLOLOLOLOLOL FAIL KIDS ARE FAILwhat this stupid answer actually means, is thatSi has 4 valence electronsCl has 7 valence electrons= 32 valence electrons in total....... Cl....... lCl - Si - Cl...... l...... ClThe bonds take up 8 electrons so 32-8= 24 electrons left.Add 6 electrons around each chlorine (6 x 4) and there are no electrons left.

3. [PDF] Outline Unit 4 – Chemical Bonding & Molecules
It's what holds ionic compounds together. 5. a. Indicate the bond type for each compound b. Draw a Lewis dot structure for each of the following: ... SiCl3Br a) ...
4. is SiCl3Br non polar or polar? - Numerade
Jul 5, 2021 · Is the molecule HOCl polar or nonpolar? Use a Lewis dot structure to explain your thinking.
VIDEO ANSWER: Hello students let's discuss the question so here we have a compound S -I -C -L -3 BR so we need to find whether this compound is polar or non -p…
5. Draw the most stable Lewis structure for NO3-. Describe its molecular ...
The formula shown represents the compound (covalent) anion of nitrate. The nitrogen atom is central. Based on their main group positions, the starting ...
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation.
6. What is the molecular geometry for SiCl3Br? - Answers
Apr 28, 2022 · The molecular geometry of carbon tetra bromide is tetrahedral. What is the molecular shape of ClNO? It would have electron geometry trigonal ...
Tetrahedral

7. Draw a Lewis structure for each of the following molecules, and then use ...
Jun 6, 2019 · SiCl3Br f. ONCl. Draw a Lewis structure for each of the following molecules, and then use the VSEPR theory to predict the molecular geometry ...
VIDEO ANSWER: The first compound is a Salford with two chlorine. Therefore so for will be the central Adam have to. Chlorine is coming off of it and then it wi…
8. Molecular Geometry: Using VSEPR Theory to Determine Three ...
For molecules or ions with three or more bonded atoms, the Lewis structure indicates which specific atoms are bonded together and the number of bonds between ...
NSHS Home Top of Page Periodic Table
9. Determine the Lewis Dot Structure for the following ion:SCl42+. - Pearson
Determine the Lewis Dot Structure for the following ion:SCl42+. A. B. C. D. 1039. views. 2. rank. Show Answer. Previous problem. Next problem. Comments (0)
Determine the Lewis Dot Structure for the following ion:SCl42+.

10. Draw the Lewis structure for SiCl4. How many bonds are around the ...
The compounds which are formed due to sharing of electrons are covalent compounds. For such covalent compounds, a Lewis structure can be drawn to show the ...
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation.
11. Determine the Lewis Dot Structure for the silicon tetrabromide mo...
Using Lewis symbols and Lewis structures, diagram the formation of BF3 from B and F atoms, showing valence-shell electrons. (c) How many valence electrons ...
Determine the Lewis Dot Structure for the silicon tetrabromide molecule, SiBr4.
